Synergistic effect p-phenylenediamine and n n-diphenylthiourea on the electrochemical corrosion behaviour of mild steel in dilute acid media
Electrochemical studies of the synergistic effect of p-phenylenediamine and n n-diphenylthiourea (TPD) as corrosion inhibitor of mild steel in dilute sulphuric and hydrochloric acid through weight loss and potentiodynamic polarization at ambient temperature were performed. Experimental results showed the excellent performance of TPD with an optimal inhibition efficiency of 88.18 and 93.88 % in sulphuric and 87.42 and 87.15 % in hydrochloric acid from both tests at all concentration studied. Polarization studies show the compound to be a mixed-type inhibitor. Adsorption of deanol on the steel surface was observed to obey the Langmuir and Frumkin isotherm models. X-ray diffractometry confirmed the absence of corrosion products and complexes. Optical microscopy confirmed the selective inhibition property of TPD to be through chemical adsorption on the steel surface.
Carbon steel is extensively utilized in petrochemical plants, chemical processing plants, extractive industries, and construction and automobile industries due to its good mechanical, chemical and physical properties. These steels are exposed to the deteriorating effect of acids in a variety of different ways resulting in corrosion.This anomaly demands the perpetual search for more effective and versatile corrosion inhibiting compounds, due to the differential environmental conditions encountered in industry. This remains a centrepiece in corrosion prevention as inhibitors decelerate the electrochemical processes responsible for corrosion. The application of inhibitors is one of the most cost effective methods for corrosion control in acidic media.
Thiourea derivatives and p-phenylenediamine have been studied individually in previous research for corrosion inhibition properties with mixed results; however, this research aims to study the synergistic effect of n n-diphenylthiourea and p-phenylenediamine as corrosion inhibitor for low carbon steel in 1 M sulphuric and 0.5 M hydrochloric acid.
Inhibiting compound
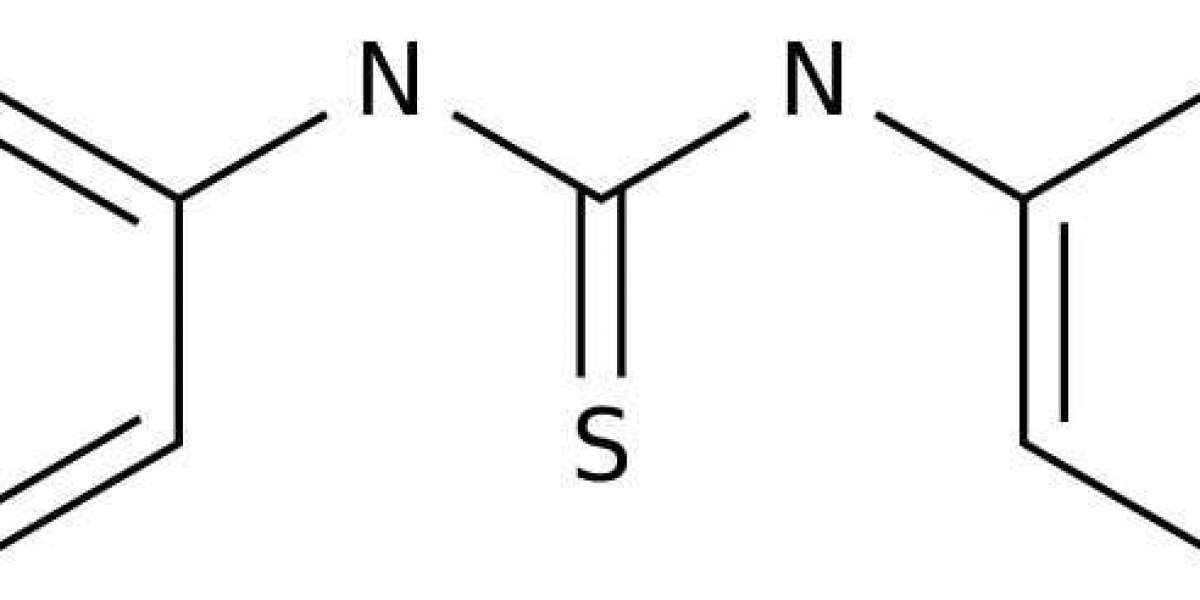
Combined mixture of n n-diphenylthiourea and p-phenylenediamine in equal proportions resulting in a whitish, solid powder (TPD) is the inhibitor used. n n-Diphenylthiourea was obtained from Sigma-Aldrich, St. Louis, USA, and p-phenylenediamine was obtained from Merck Chemical, Germany. The structural formula of n n-diphenylthiourea is shown in Fig. 1a, the molecular formula is C13H12N2S, while the molar mass is 228.312 g/mol. The molecular formula p-phenylenediamine is C6H4(NH2)2, while the molar mass is 108.1 g/mol.
Study of previous research on p-phenylenediamine in HCl and H2SO4 shows that desorption occurs at very low and higher concentrations of the organic compound due to lateral repulsion between the inhibitor molecules which results in weak inhibitor covering over the steel surface and hence significant increase in corrosion rate. Observation of data from unpublished research identifies similar phenomenon for n n-diphenylthiourea in HCl. The combined action/synergistic effect of the compounds in TPD displayed remarkable improvement in corrosion inhibition and inhibition efficiency.