Helicobacter pylori (HP) is a Gram-negative, helix-shaped, microaerophilic bacterium that belongs to the family Helicobacteraceae, genus Helicobacter. It is usually found in the stomach. Pathogenesis of Helicobacter pylori depends on the fact that it can survive in the harsh gastric environment. This environment is thought to be an impossible zone for bacterium before, as it is characterized by peristalsis, acidity, and attack by phagocytes and released reactive oxygen species. It also plays a role in the development of stomach cancer and duodenal ulcers. More than half of the world's population harbor this bacterium in their upper gastrointestinal tract. But nearly 85% of them never experience any symptoms or complications. Individuals who were infected with Helicobacter pylori have a risk of developing peptic ulcers by 10% to 20%, and acquiring stomach cancer by about 1% to 2%.
Helicobacter pylori is a helix-shaped bacterium with a length of about 3 μm and a diameter of about 0.5 μm. It is microaerophilic, which means that it requires oxygen but at a lower concentration than that of the atmosphere. Helicobacter pylori consists of a large amount of strains with relatively large difference in their genomes. Its genome contains about 1.7 million base pairs, which encode more than 1500 genes. Helicobacter pylori possesses five major outer membrane protein (OMP) families, including porins, iron transporters, flagellum-associated proteins, known and putative adhesins and proteins of unidentified function. OMP of Helicobacter pylori contains lipopolysaccharide (LPS) and phospholipids, as well as other typical Gram-negative bacteria.
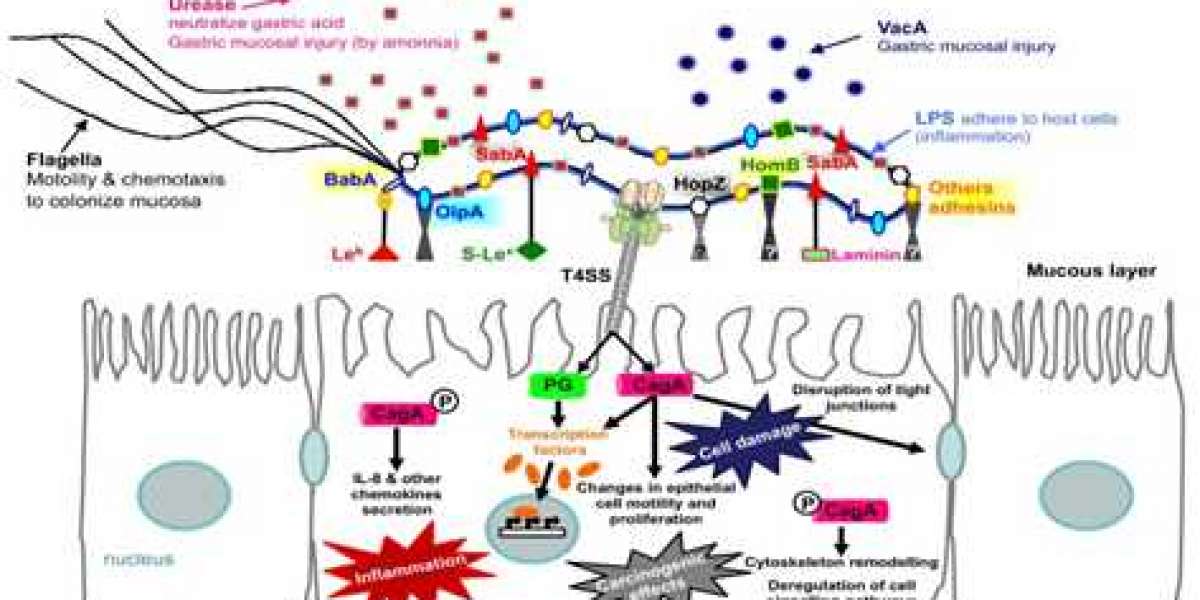
After entering the host stomach, H. pylori utilizes its urease activity to neutralize the hostile acidic condition at the beginning of infection. Flagella-mediated motility is then required for H. pylori to move toward host gastric epithelium cells, followed by specific interactions between bacterial adhesins with host cell receptors, which thus leads to successful colonization and persistent infection. Finally, H. pylori releases several effector proteins/toxins, including cytotoxin-associated gene A (CagA), and vacuolating cytotoxin A (VacA), causing host tissue damage.
- Cytotoxin-associated gene A (CagA) CagA can alter host cell signaling in both a phosphorylation-dependent and phosphorylation-independent manner. The phosphorylated CagA binds to the phosphatase SHP-2 and affects the adhesion, spreading, and migration of the cell. Moreover, CagA can also affect the host cell in several aspects, such as the formation of gastric epithelium cell pedestals, the change of the cytoskeleton, affecting the proliferation of cells, and stimulating the gastric epithelium cells to secrete IL-8.
- Vacuolating cytotoxin A (VacA) The secreted VacA toxin is composed of the p33 and p55 domains that form an oligomeric structure. This complex can embed into the host cell membrane and help H. pylori colonization. This complex can also get into the endosome via endocytosis. VacA induces the release of cytochrome C, ER stress, and apoptosis. In addition, VacA disrupts the balance of cell proliferation and death by affecting genes that regulate the cell cycle. It also can induce acute inflammatory responses through inducing host cell release of IL-8.
- Heat Shock Proteins (HSP) H. pylori produces mainly two Hsps, GroES-like HspA (Hsp10), and GroEL-like HspB (Hsp60). The high expression of Hsp60 at low pH, which interacts with the receptor-like sulfatide (sulfoglycolipid), indicates the stress of acid may change the specificity of H. pylori to receptors. Hsp60 induces activation of NF-kB via TLR2 and the mitogen-activated protein kinase pathway, and thereby induces human monocytes to secrete IL-8.
- Outer Membrane Proteins (OMP) In 1997, the genome of H. pylori strain 26695 was sequenced completely, and it was suggested to encode 32 OMPs. Sialic acid-binding adhesin (SabA), blood-group-antigen-binding adhesin (BabA), adherence-associated lipoprotein A and B (AlpA/B), outer inflammatory protein A (OipA), and Helicobacter outer membrane protein Q (HopQ) were involved in adhesion. OMPs are important for colonization and the establishment of inflammation in the stomach.
- Urease proteins Helicobacter pylori urease is composed of two structural proteins, α and β subunits, where the β subunit is 60 kDa and the α subunit is approximately 30 kDa. Urease is central to H. pylori metabolism and virulence, is necessary for its colonization of the gastric mucosa, and is a potent immunogen that elicits a vigorous immune response.
With years of protein production experience and state-of-art facilities, Creative Diagnostics now can provide multiple high-quality Helicobacter pylori antigens to our customers with a cheaper price for various applications in both industry and academic programs. Welcome to contact us for more details.