Irritation represents local tissue inflammation response to chemicals, without a systemic immunological component, and irritation is characterized by inflammation, redness, swelling, heat, or pain. Irritation testing is used to assess the potential of a medical device to cause an immediate irritation reaction in the skin, mucosal, or ocular tissues following a single or repeated exposure to the body. Irritation is one of the three most common biocompatibility tests required to ensure the safety of medical devices.
Standard for Irritation Testing
Irritation testing is instructed by FDA regulations and international standards. “Assessing the Irritation and Sensitization Potential of Transdermal and Topical Delivery Systems for ANDAs”, introduced by the FDA as guidance for industry, provides guidance for the design and carry out of studies to assess the potential of in vivo skin irritation and sensitization induced by a medical device.
“Tests for irritation and skin sensitization”, part ten of the Biological evaluation of medical devices standards (ISO 10993-10), gives out the general considerations that should be taken into account when evaluating the potential of inducing irritation of a medical device. It outlines the procedure of studies to assess the potential of medical devices and their constituent materials to induce irritation and skin sensitization.
STEMart offers several methods as in vivo and in vitro assays for irritation biocompatibility testing following the biocompatibility guidelines modified for medical devices. The testing is designed and performed based on the route of exposure to the body including the skin, mucosal, or ocular.
Irritation Testing Capabilities
In the Topical Irritation Testing, the erythema and edema (redness and swelling) on the injection site are assessed after injection of extracts of the test material. It is suitable for the detection of the potential for local irritation caused by chemicals extracted from a biomaterial.
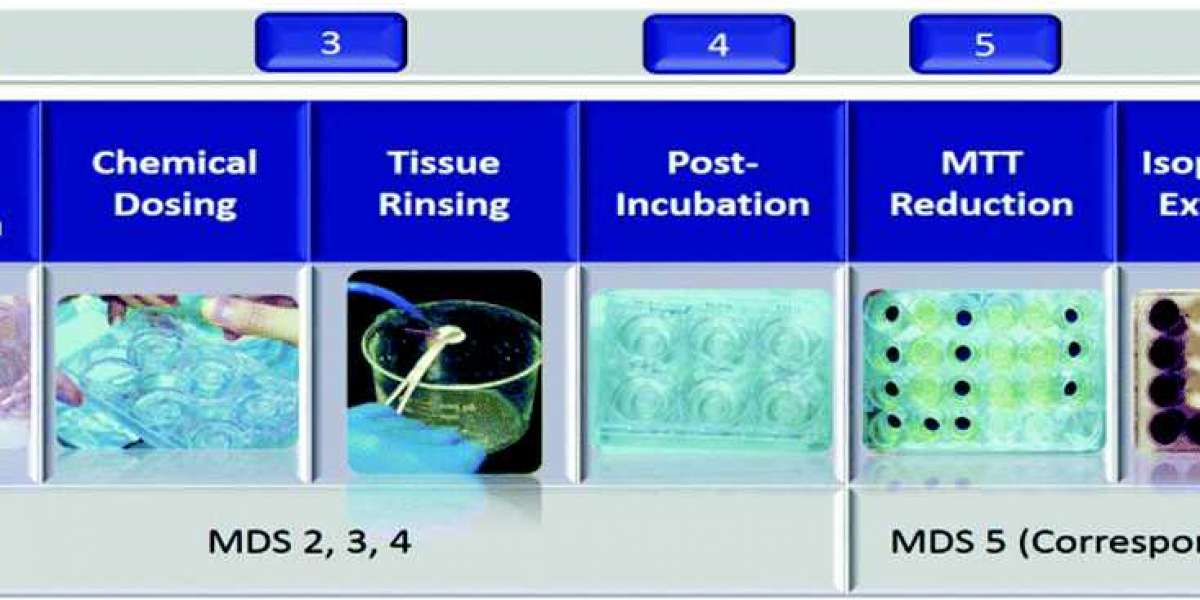
Skin Irritation Testing
Since the skin irritation is always caused by chemical-induced cell damage and subsequent inflammatory cascade, our skin irritation testing measures cell viability in reconstructed human epidermis (RhE) tissues after a single chemical exposure.

Fig. 1 Procedures of skin irritation test (SIT). (Lin, 2019)
Ocular Irritation Testing
We provide several in vitro test methods for the identification of ocular irritation to satisfy the specific medical device requirements, including bovine corneal opacity and permeability (BCOP), the isolated chicken eye (ICE) test methods, Cytosensor microphysiometer (CM) test method, chorioallantoic membrane (HET-CAM) test method and isolated rabbit eye (IRE) test method. In vivo test is also provided for weak irritants.
- Oral Irritation
- Vaginal Irritation
- Nasal Irritation
- Penile Irritation
- Rectal Irritation
In the Intracutaneous Reactivity Testing, the erythema and edema (redness and swelling) on the injection site are assessed after injection of extracts of the test material. It is suitable for the detection of the potential for local irritation caused by chemicals extracted from a medical device.
If you have additional questions about Irritation Testing or would like to find out more about our services, please feel free to contact us.
Reference
- Lin, Yu-Chun, et al.“Testing Method Development and Validation for in Vitro Skin Irritation Testing (SIT) by Using Reconstructed Human Epidermis (RhE) Skin Equivalent-EPiTRI®.” Alternatives to Animal Testing 8-19 (2019).